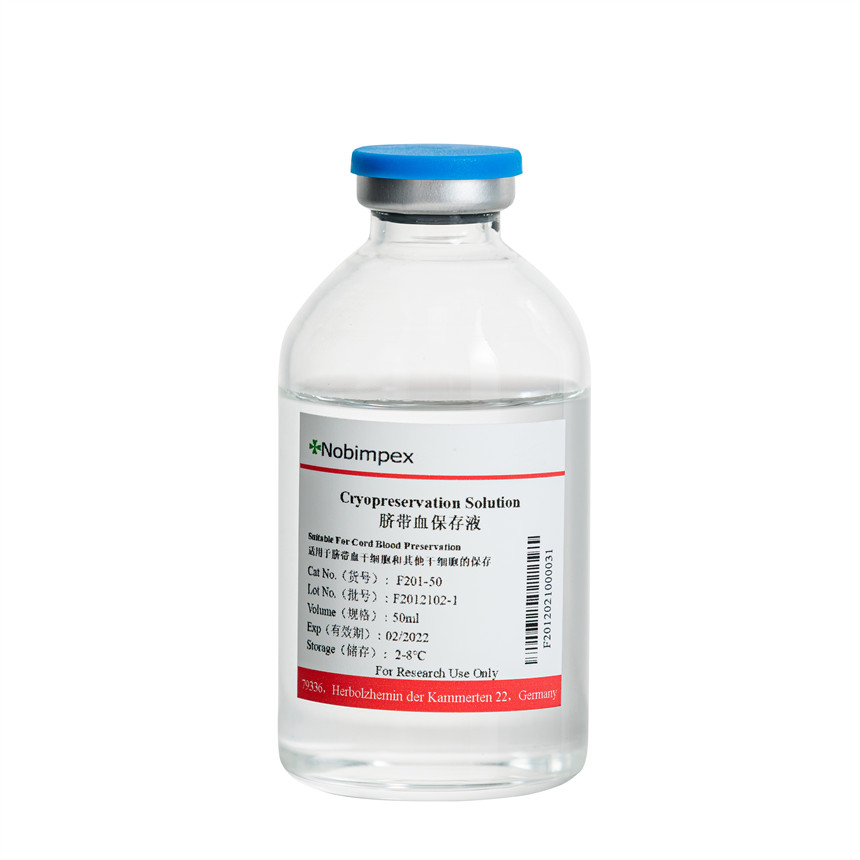
The cord blood cryopreservation solution is produced according to cGMP regulations, is CFDA-filed and culture-validated. The production uses disposable medical consumables to effectively avoid cross-contamination. It is mainly used for the preservation of cord blood stem cells and other stem cells.
The main ingredients of this cryopreservation solution are 55% dimethyl sulfoxide (DMSO) and 5% dextran. Raw material sources are certified to meet USP Grade standards.
Application:Suitable for the preservation of cord blood stem cells and other stem cells.
Chemically defined product.
The raw materials are imported from USA and in compliance with the USP Grade standards.
Production according to cGMP regulations.
Sterile, endotoxin and pyrogen-free, mycoplasma testing, and cell culture testing.
Instructions for use: