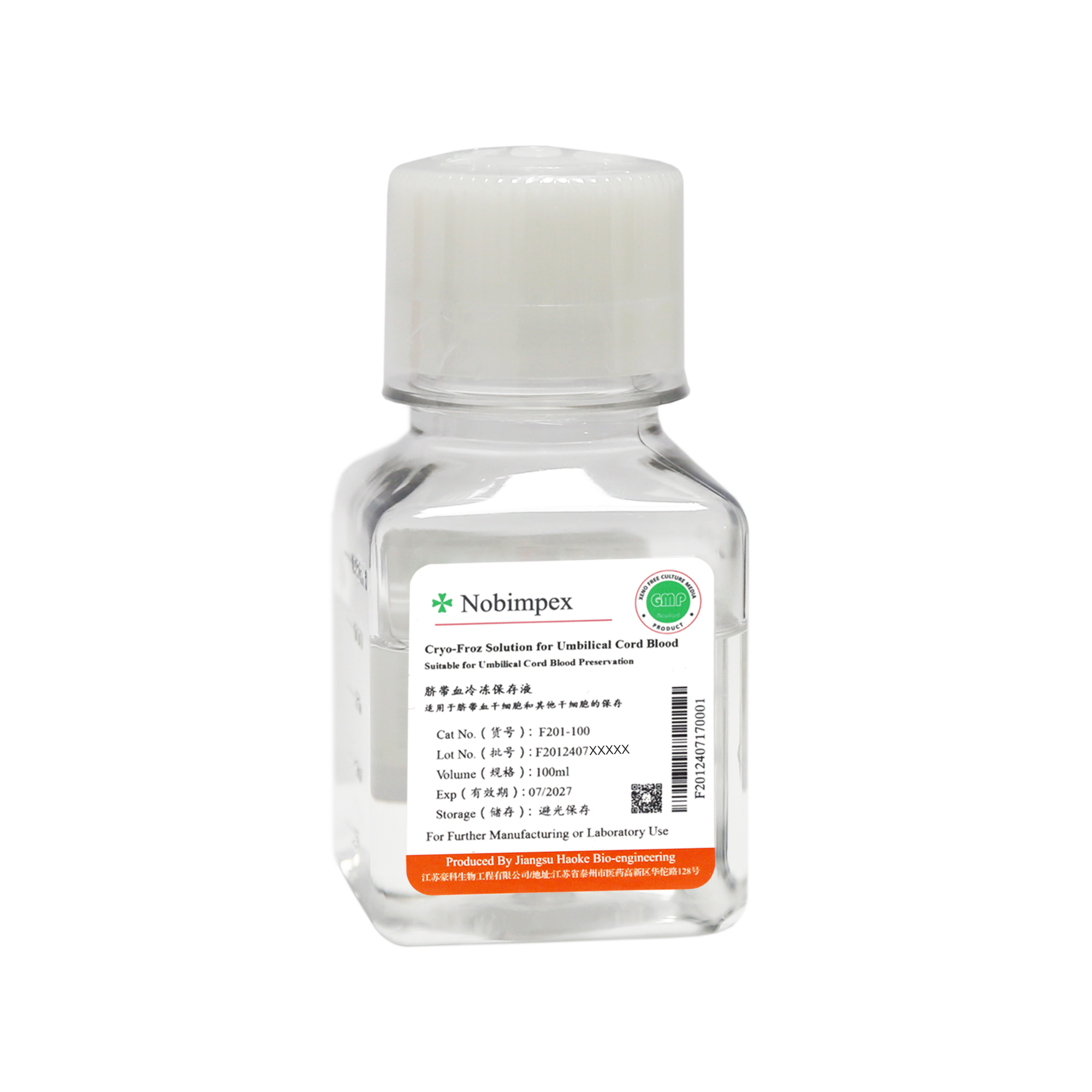
The cord blood cryopreservation solution is produced according to cGMP regulations, is CFDA-filed and culture-validated. The production uses disposable medical consumables to effectively avoid cross-contamination. It is mainly used for the preservation of cord blood stem cells and other stem cells.
The main ingredients of this cryopreservation solution are 55% dimethyl sulfoxide (DMSO) and 5% dextran. Raw material sources are certified to meet USP Grade standards.
Application:Suitable for the preservation of cord blood stem cells and other stem cells.
Instructions for use: